
Nerve Protection that Changes the Game
Tulavi is redefining peripheral nerve surgery with proven hydrogel technology designed to optimize nerve recovery.


Tulavi is redefining peripheral nerve surgery with proven hydrogel technology designed to optimize nerve recovery.

When a peripheral nerve is severed, regenerating axons may grow erratically, resulting in the development of a painful neuroma.What is a Neuroma? A neuroma is a non-cancerous growth of nerve tissue that commonly arises after a nerve has been injured or disrupted.
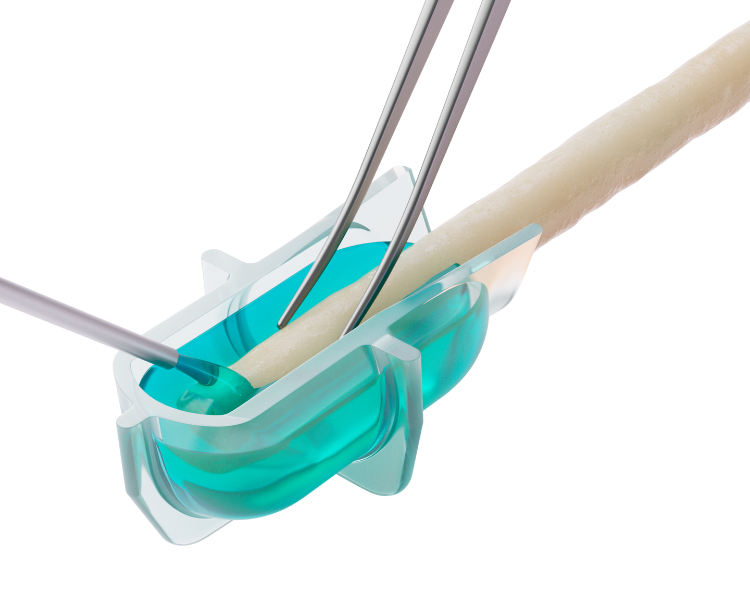
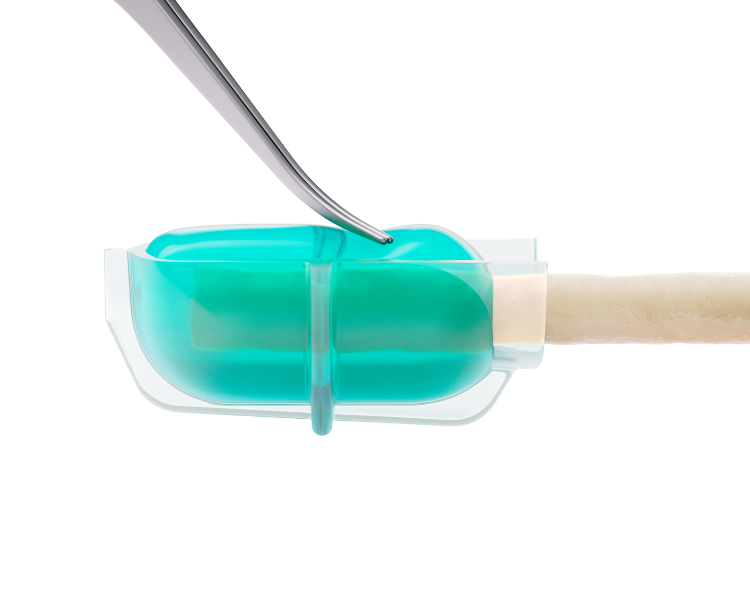
The fully-absorbable allay™ Hydrogel Cap is designed to protect the transected nerve, optimizing recovery and reducing the risk of neuroma formation.
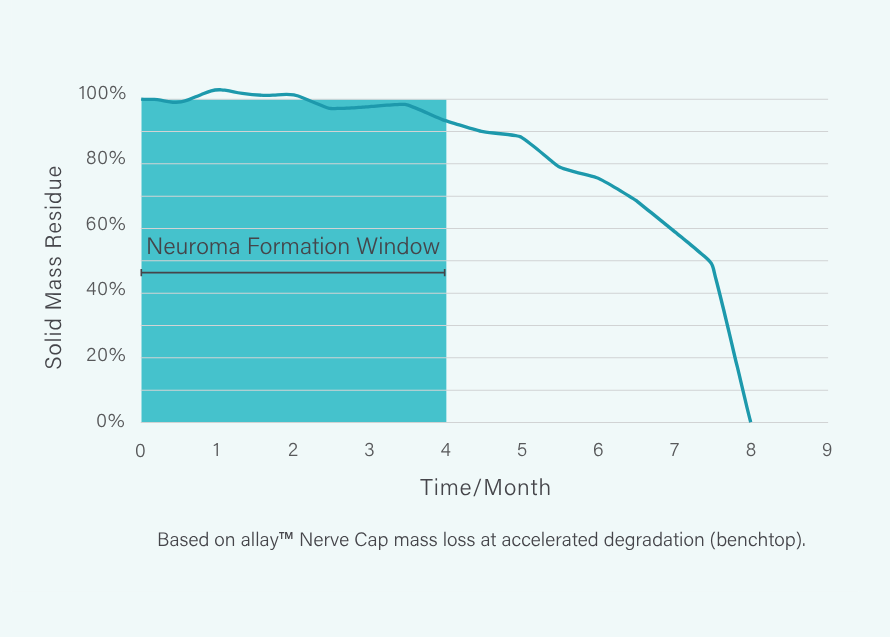
allay™ Hydrogel Cap remains in place for 8 months, protecting the nerve throughout the critical period of neuroma formation.²,³
Built on the Adaptive Hydrogel Technology, which has been used in over 5 million patients worldwide⁴, the fully absorbable allay™ Hydrogel Cap is designed to:
Provides a mechanical barrier, preventing escape of disorganized axons
No neuroma formation demonstrated across 6 preclinical studies, n>170
The allay™ Hydrogel Cap rapidly forms in situ to create a conformable and protective cushion.

A single allay™ Hydrogel Cap Set enables surgeons to efficiently treat multiple nerves across a broad range of sizes.
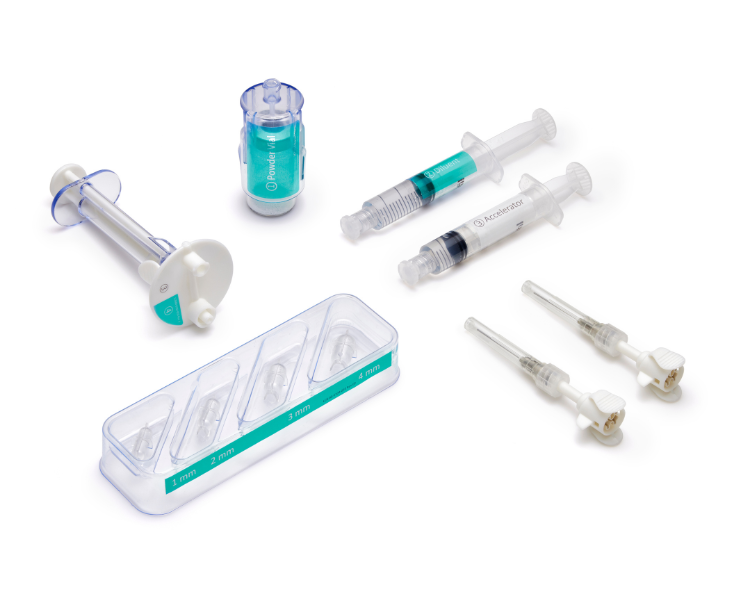
Small Nerve Set
Nerve Sizes: 1, 2, 3 and 4 mm
Model # TL-5515-1
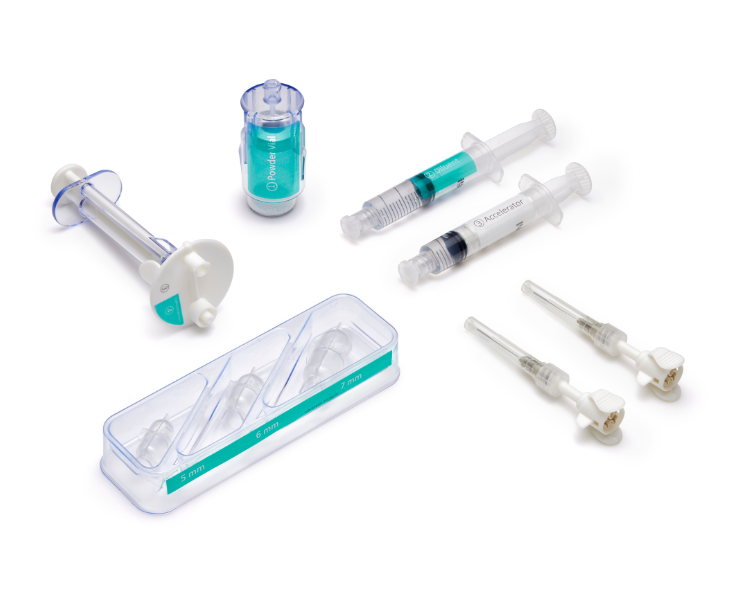
Large Nerve Set
Nerve Sizes: 5, 6, and 7 mm
Model # TL-5515-2
Additional Delivery Tips
One box includes 6 packages
Each package contains one single use delivery tip
Model # TL-7627
The allay™ Nerve Cap is indicated for use in individuals weighing 25 kg or more as a physical barrier to separate the peripheral nerve end from the surrounding environment to reduce the risk of the development of a symptomatic neuroma.
The allay™ Nerve Cap is not designed, sold, or intended for use except as described in the indication for use and is contraindicated for use in:
Potential complications that may be associated with any peripheral nerve surgery may include pain, infection, decrease or increase in nerve sensitivity, and complications associated with the use of anesthesia. Minor discomfort in the surgical site may occur for several days.
Based on product volumes from four hydrogel companies across multiple specialties, developed by Incept LLC.